Dominance of sulfur-fueled iron oxide reduction in low-sulfate freshwater sediments | The ISME Journal
![PDF] Mechanism of Metastable Wüstite Formation in the Reduction Process of Iron Oxide below 570 | Semantic Scholar PDF] Mechanism of Metastable Wüstite Formation in the Reduction Process of Iron Oxide below 570 | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/57db7b49cd7cc6ba876405b795677fa1c97a8223/1-Table1-1.png)
PDF] Mechanism of Metastable Wüstite Formation in the Reduction Process of Iron Oxide below 570 | Semantic Scholar

In an experiment, 2.4 g of iron oxide on reduction with hydrogen gave 1.68 g of iron. In another experiment, 2.69 g of iron oxide gave 1.88 g of iron on reduction.

Electrochemical Reduction of Carbon Dioxide and Iron Oxide in Molten Salts to Fe/Fe3C Modified Carbon for Electrocatalytic Oxygen Evolution - Liang - 2021 - Angewandte Chemie International Edition - Wiley Online Library

In an experiment, 2.4 g of iron oxide on reduction with hydrogen yield 1.68 g of iron. In another experiment. 2.9 g of iron oxide give 2.03 g of iron on reduction

In an experiment, 2.4 g of iron oxide on reduction with hydrogen gave 1.68 g of iron. In another experiment, 2.69 g of iron oxide gave 1.88 g of iron on reduction.

Electrochemical Analysis of Changes in Iron Oxide Reducibility during Abiotic Ferrihydrite Transformation into Goethite and Magnetite | Environmental Science & Technology

Reduction of Iron Oxides with Hydrogen—A Review - Spreitzer - 2019 - steel research international - Wiley Online Library
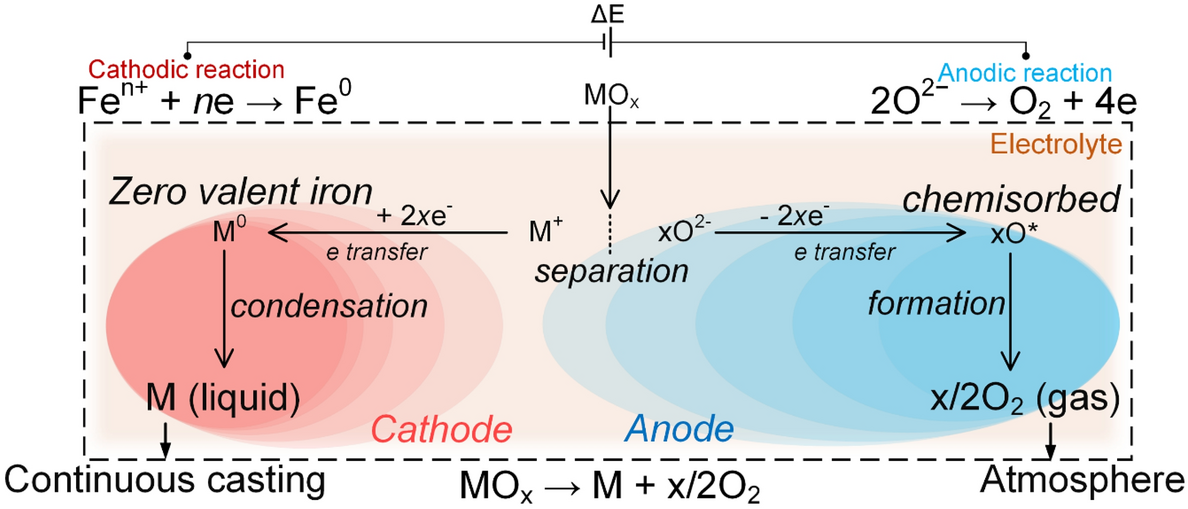
Oxidation and electrical properties of chromium–iron alloys in a corrosive molten electrolyte environment | Scientific Reports

7.95 gm oxide of copper on reaction with hydrogen shows a mass loss and the residue obtained weighs 6.35 gm. In another experiment, 19.05 gm of copper was dissolved in nitric acid