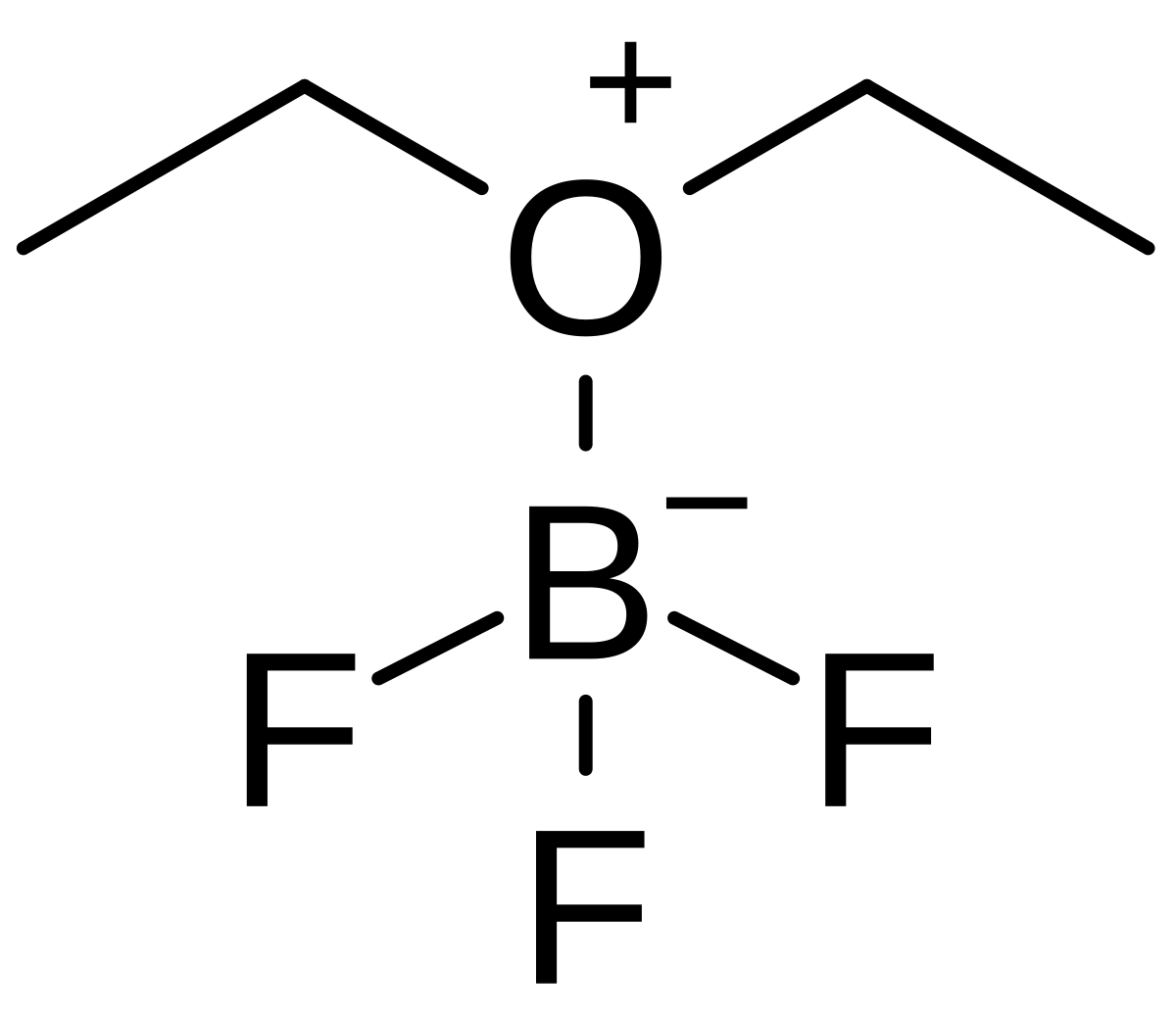
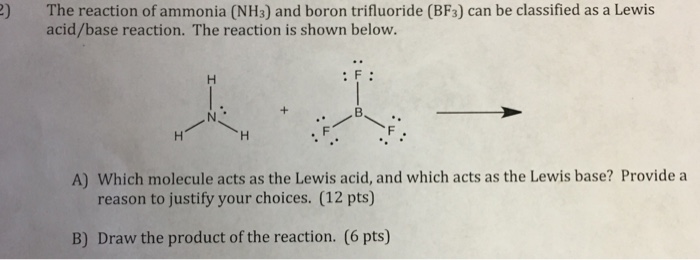
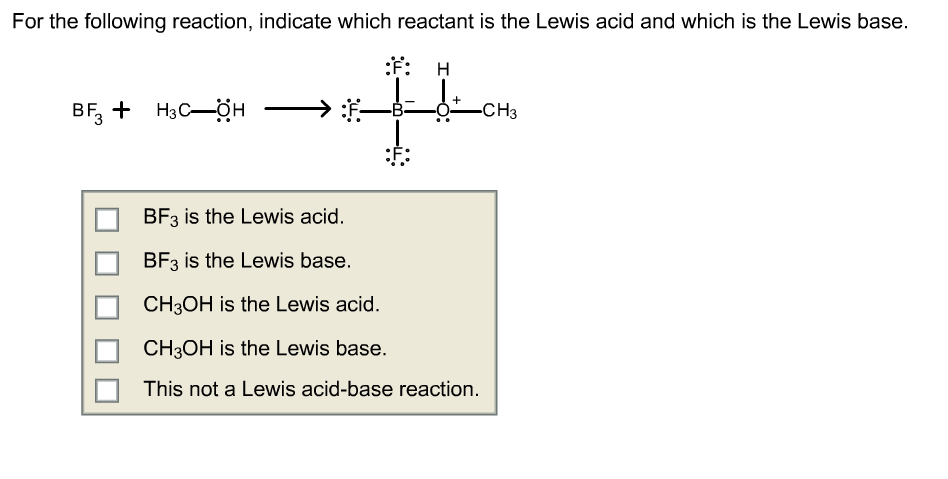
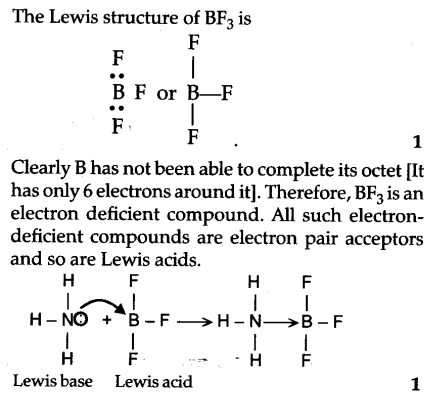
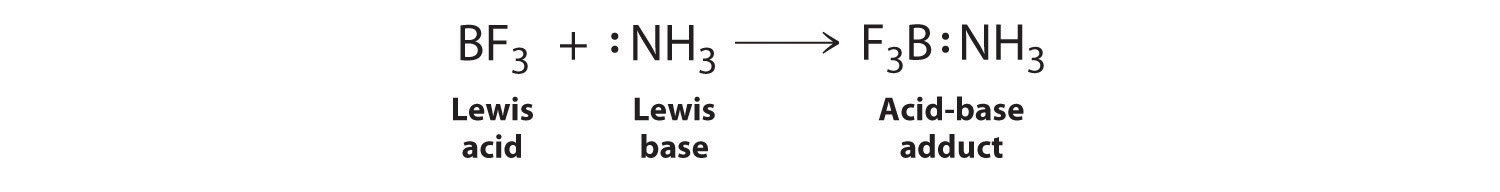
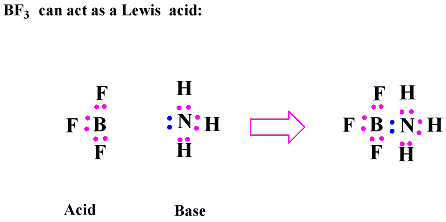
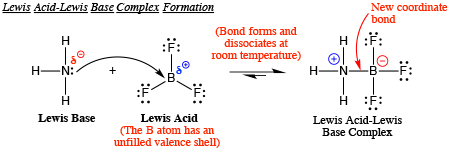
Draw structural formulas to represent the reaction between a Lewis acidand a Lewis base and identify the nature of the bond formed in the product

Why BF3 acts as Lewis Acid | Boron trifluoride acts as a Lewis Acid why | Lewis Acid and Base Theory - YouTube

Draw the product of the following Lewis acid-base reaction below. Label the electrophile and nucleophile. | Homework.Study.com
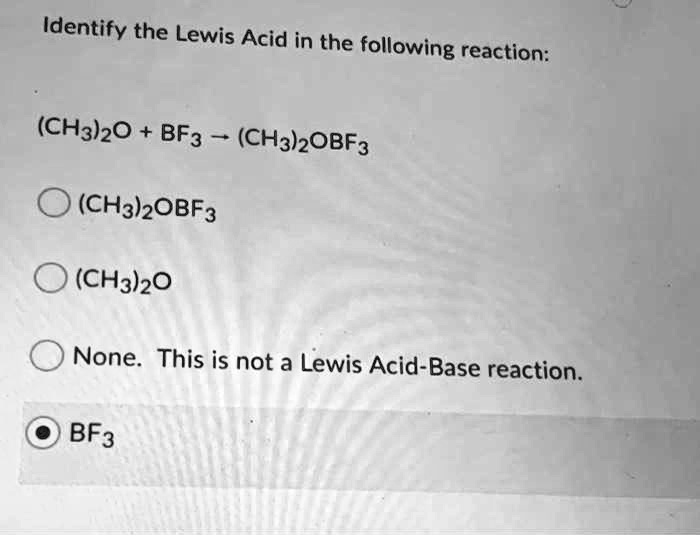
SOLVED: Identify the Lewis Acid in the following reaction: (CH3)2o BF3 (CH3)OBF3 (CH3)OBF3 (CH3h)20 None This is not a Lewis Acid-Base reaction. BF3

Draw the lewis structure of the 1:1 adduct that forms in the lewis acid-base reaction between boron - Brainly.com