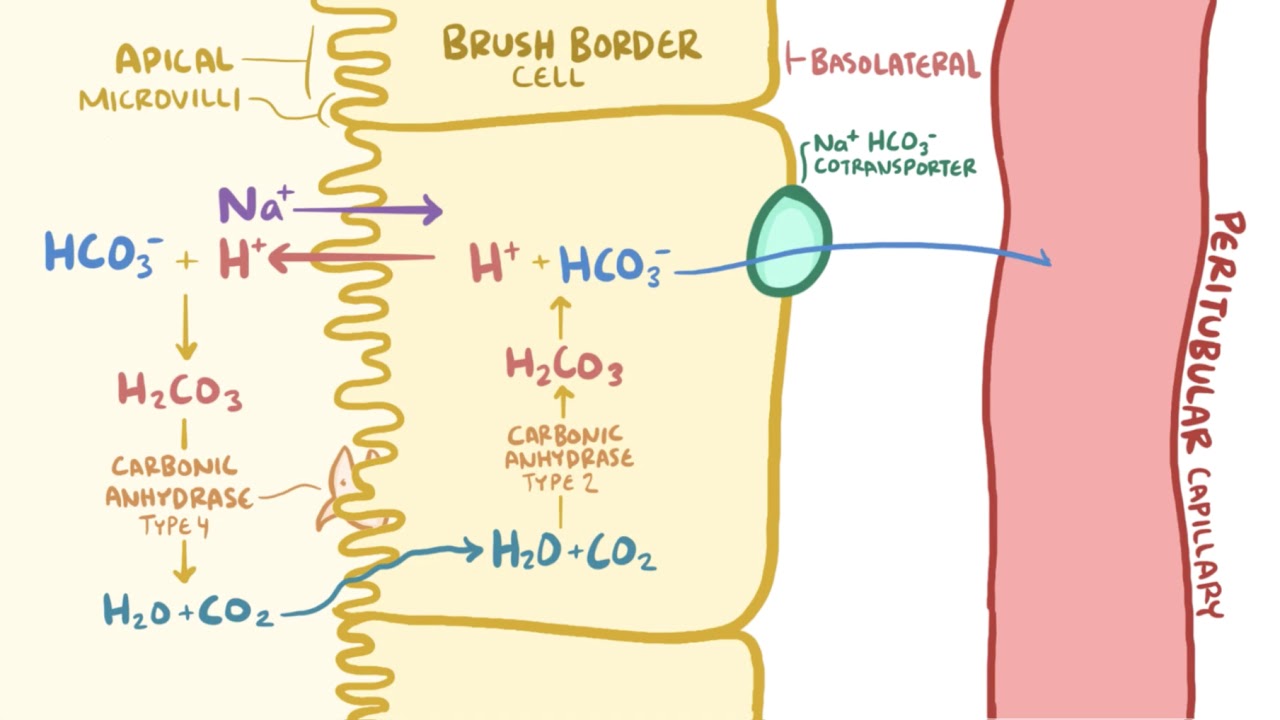
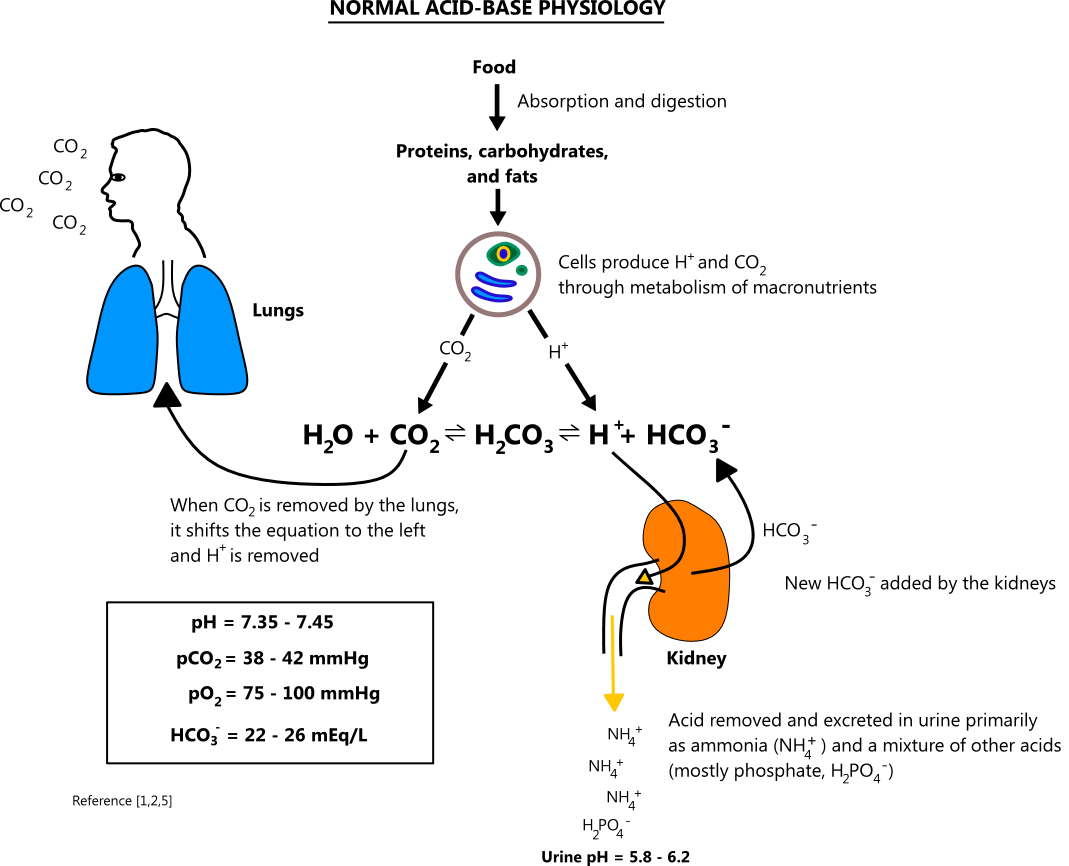
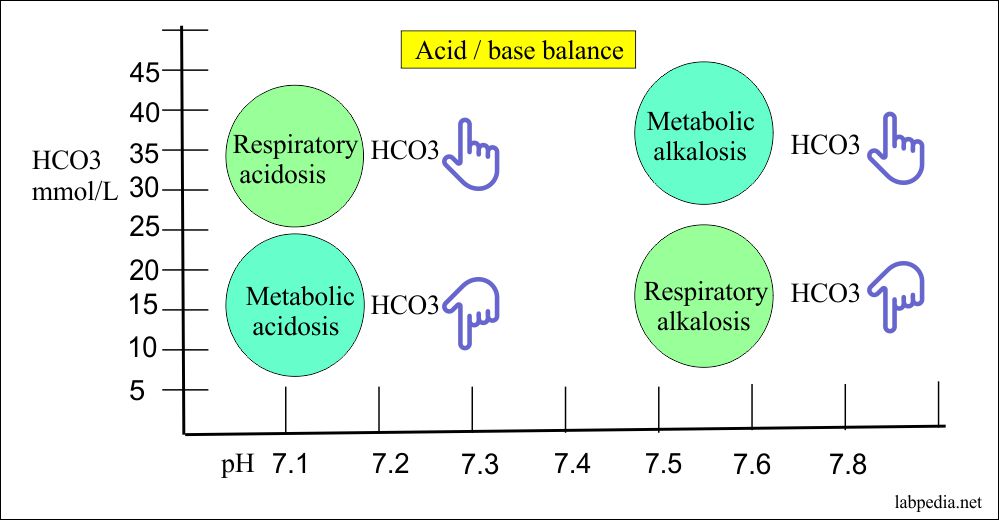
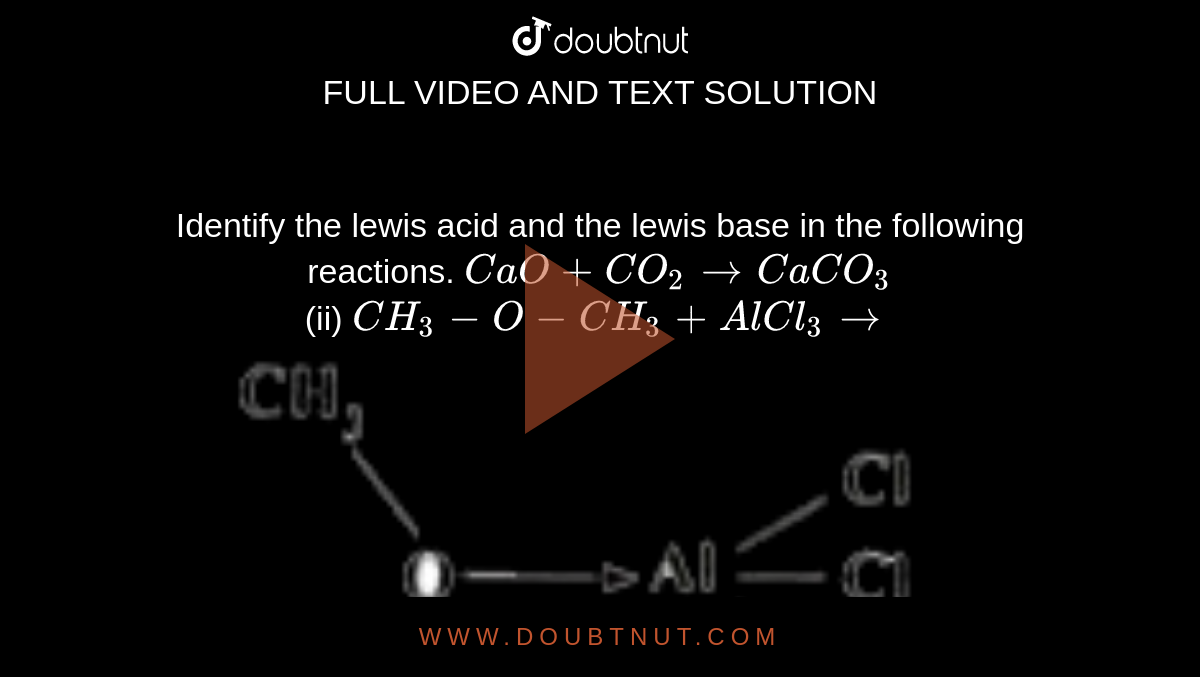
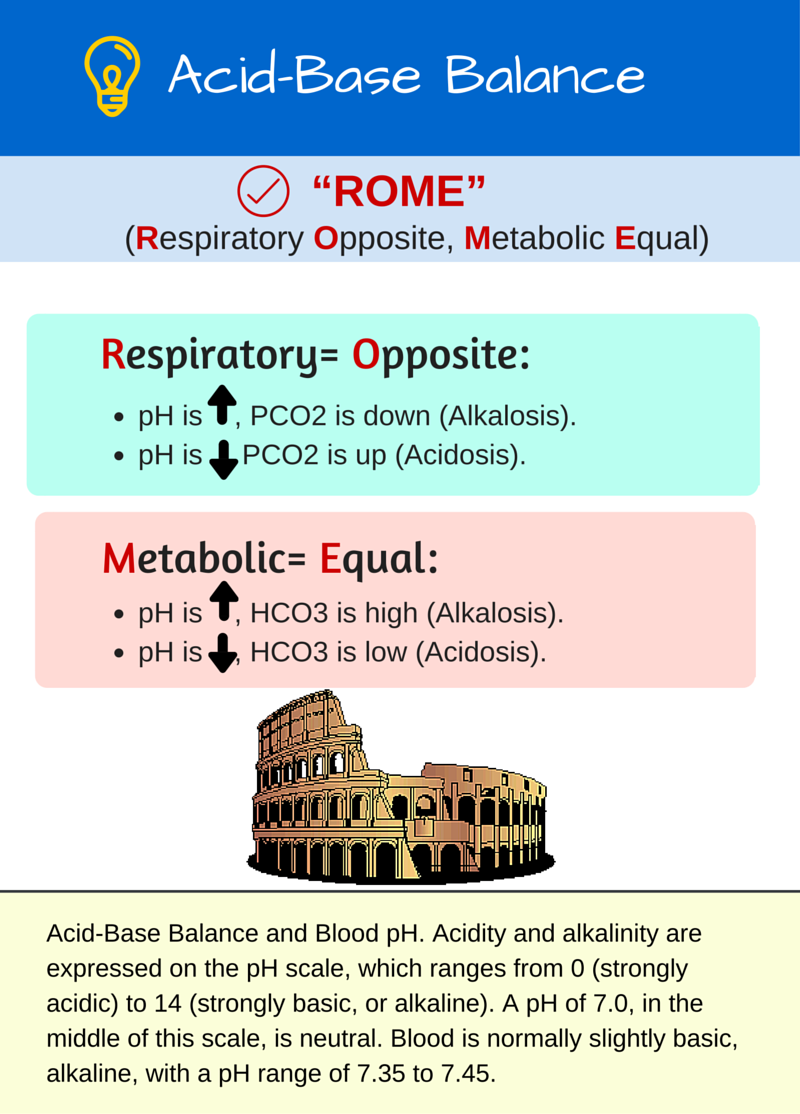
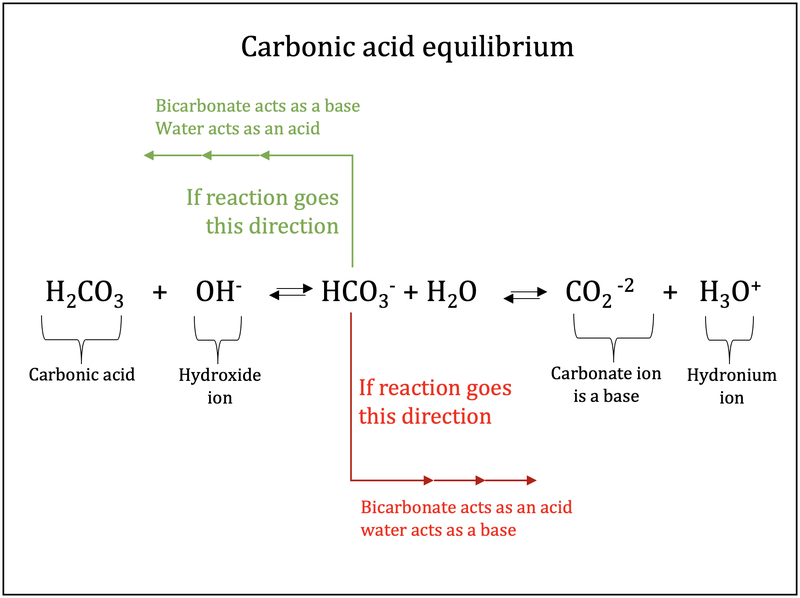
Rishi Kumar, MD - I created this table to teach my trainees how I approach acid-base problems assuming a normal bicarbonate (HCO3) of 24 mmol/L, PaCO2 40 mmHg, arterial pH 7.38-7.42, and
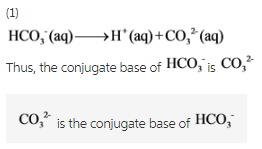
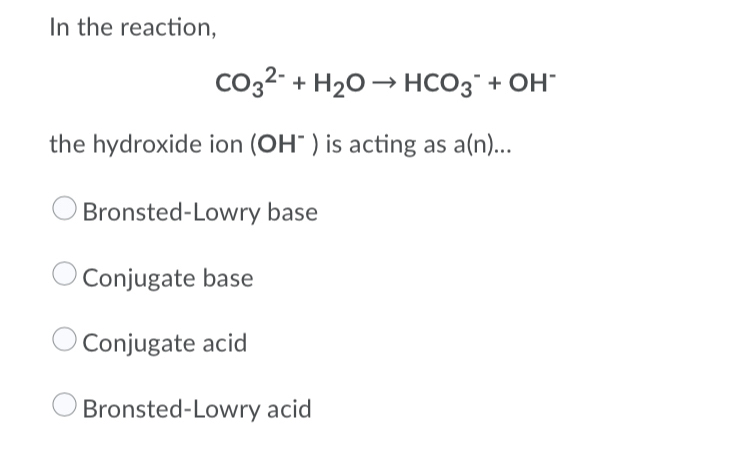
What is the conjugate base of HCO3−? Express your answer as a chemical formula - Home Work Help - Learn CBSE Forum

physical chemistry - Which make HCO3- to show two pH values at two scenarios? - Chemistry Stack Exchange
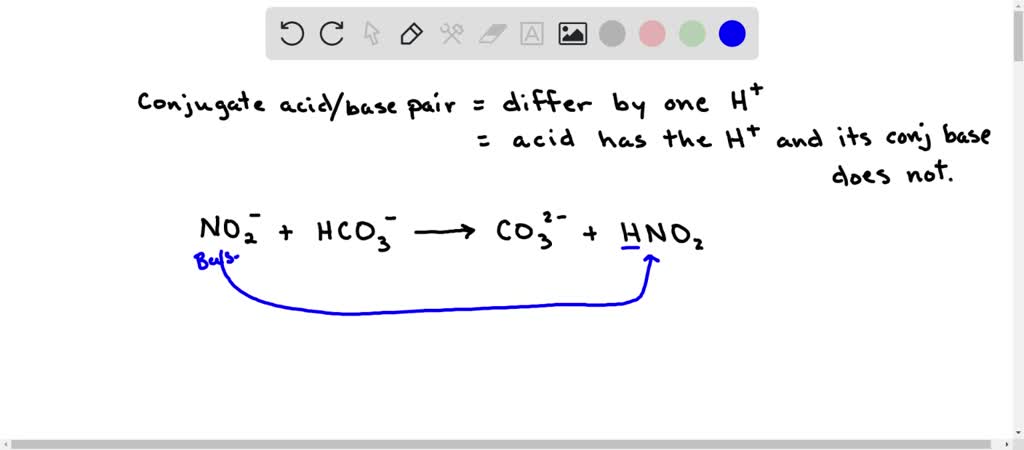
SOLVED: Consider the following reaction: NO2 - + HCO3 - ⇌ CO3 2- + HNO2 Identify the acid, base, conjugate acid and conjugate base.