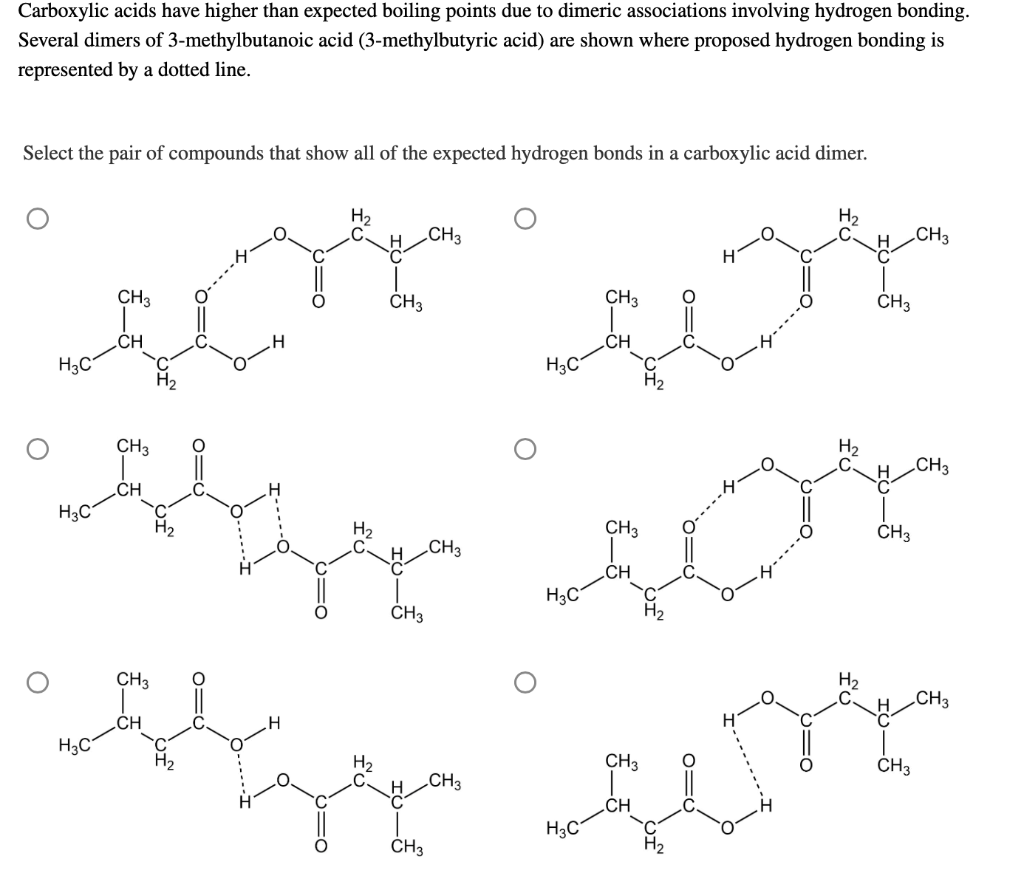
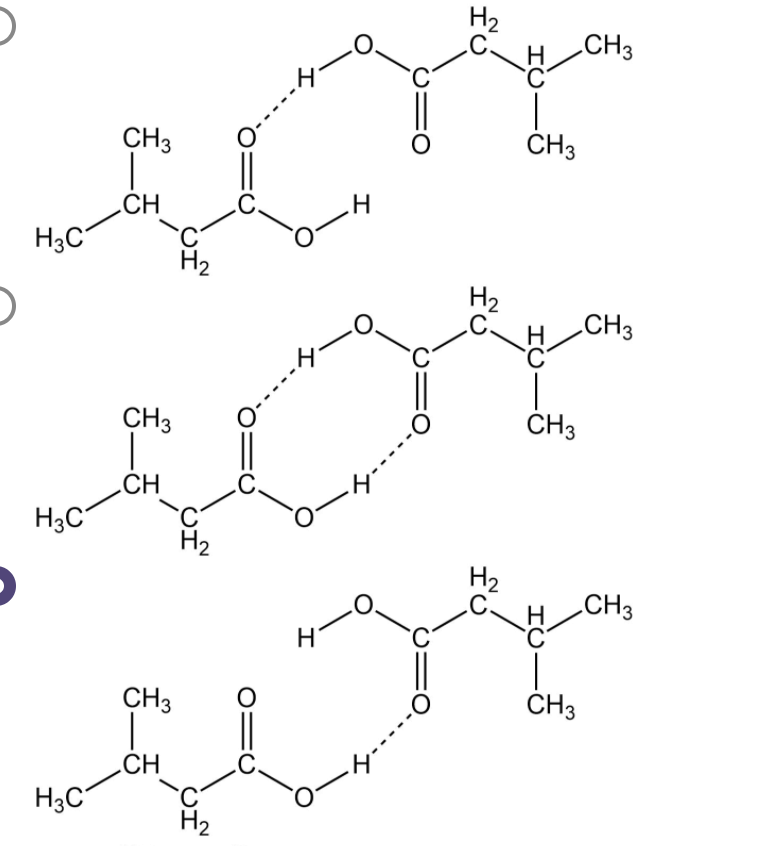
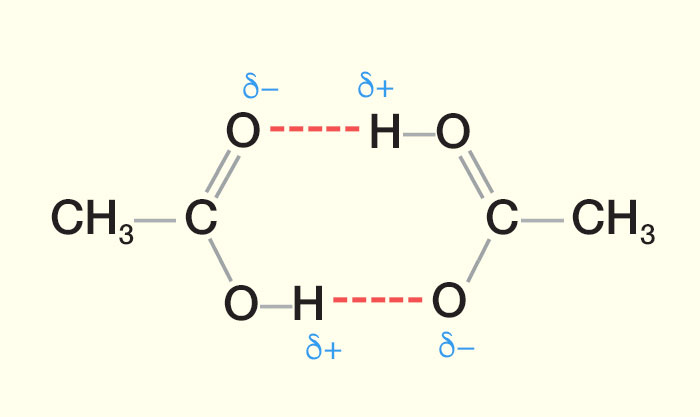
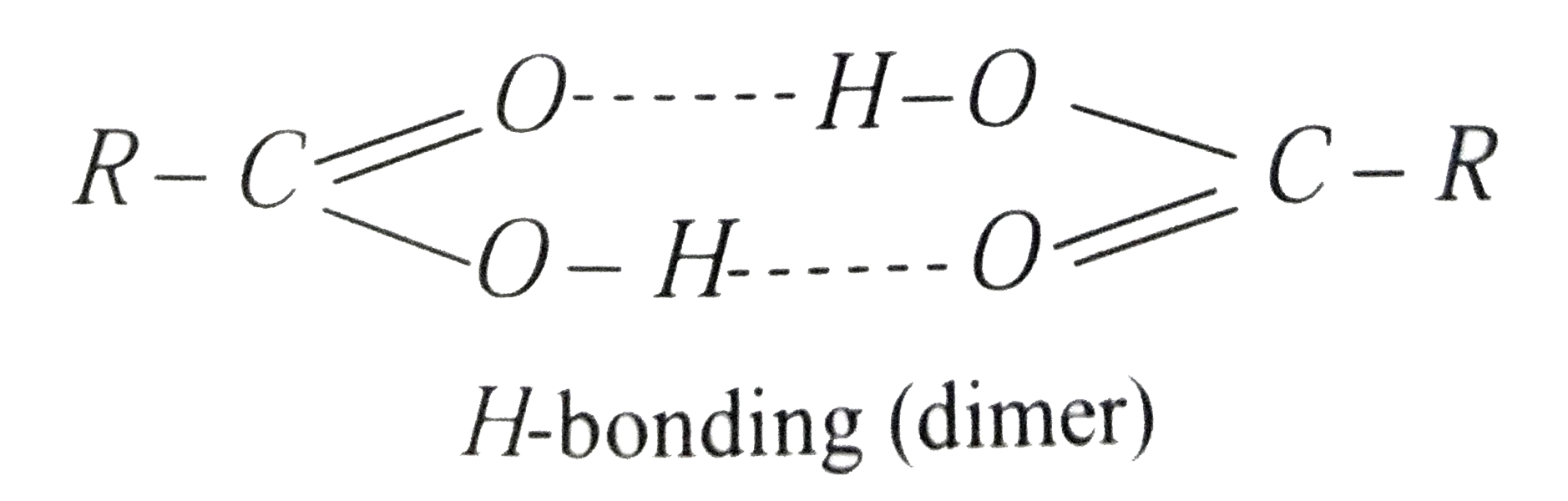
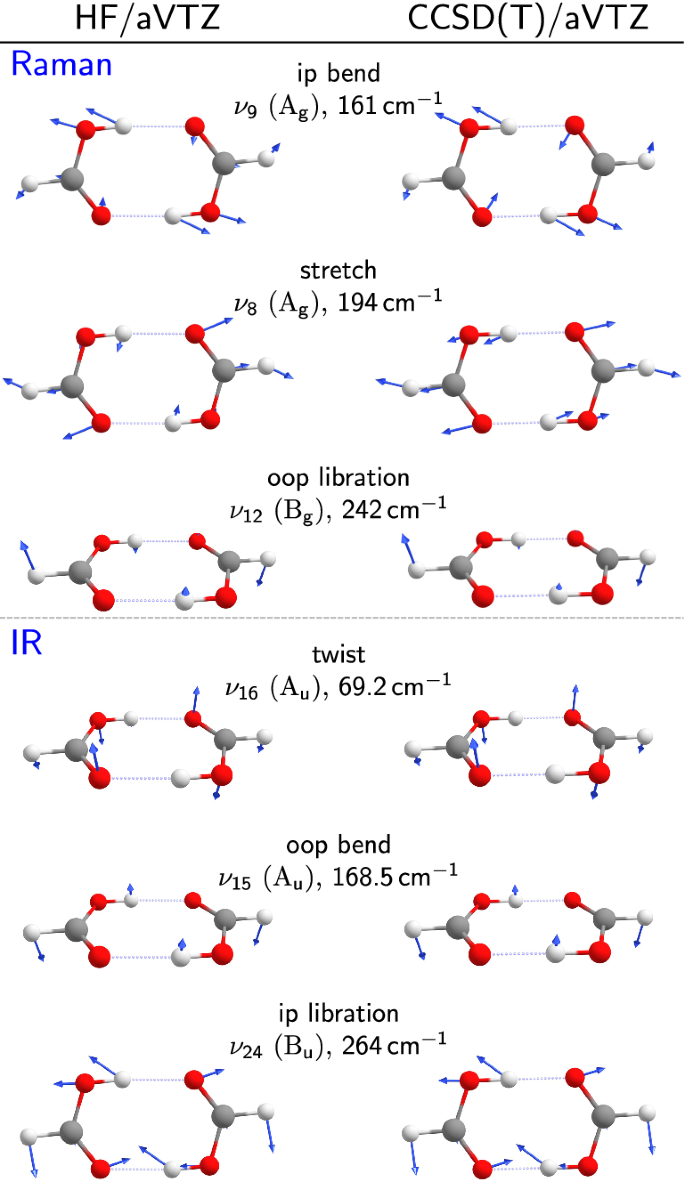
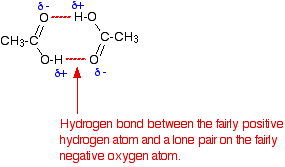
Carboxylic acids have higher than expected boiling points due to dimeric associations involving hydrogen bonding. The structure of 3-methylbutyric acid is shown below. Draw a second molecule of 3-meth | Homework.Study.com
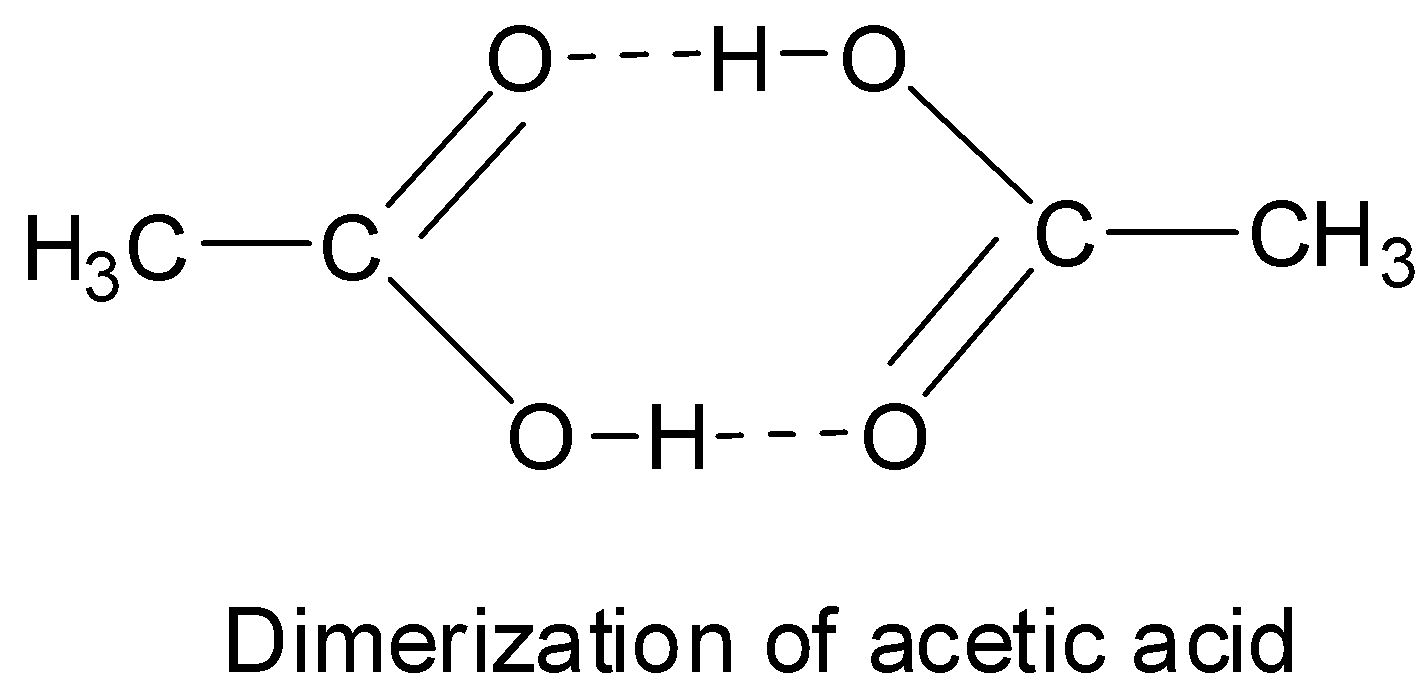
Acetic acid exists as dimer in benzene due to:A.Condensation reactionB.Hydrogen bondingC.Presence of carboxyl groupD.Presence of hydrogen atom at ${{\\alpha }}$ -carbon

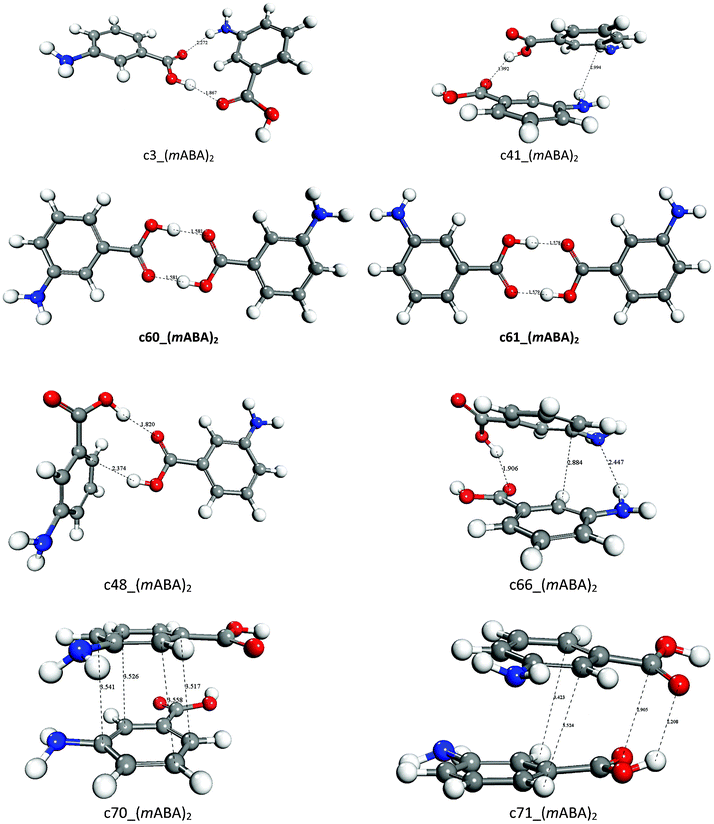
Unique homo and hetero carboxylic acid dimer-mediated supramolecular assembly: rational analysis of crystal structure of 3,5-dinitrobenzoic acid and 4-(N-methylamino)benzoic acid - ScienceDirect
The molecular self-association of carboxylic acids in solution: testing the validity of the link hypothesis using a quantum mechanical continuum solva ... - CrystEngComm (RSC Publishing) DOI:10.1039/C3CE40539G

Solid-State 17O NMR Study of Carboxylic Acid Dimers: Simultaneously Accessing Spectral Properties of Low- and High-Energy Tautomers | The Journal of Physical Chemistry A

organic chemistry - Why can't alcohols form hydrogen-bonded dimers like carboxylic acids? - Chemistry Stack Exchange