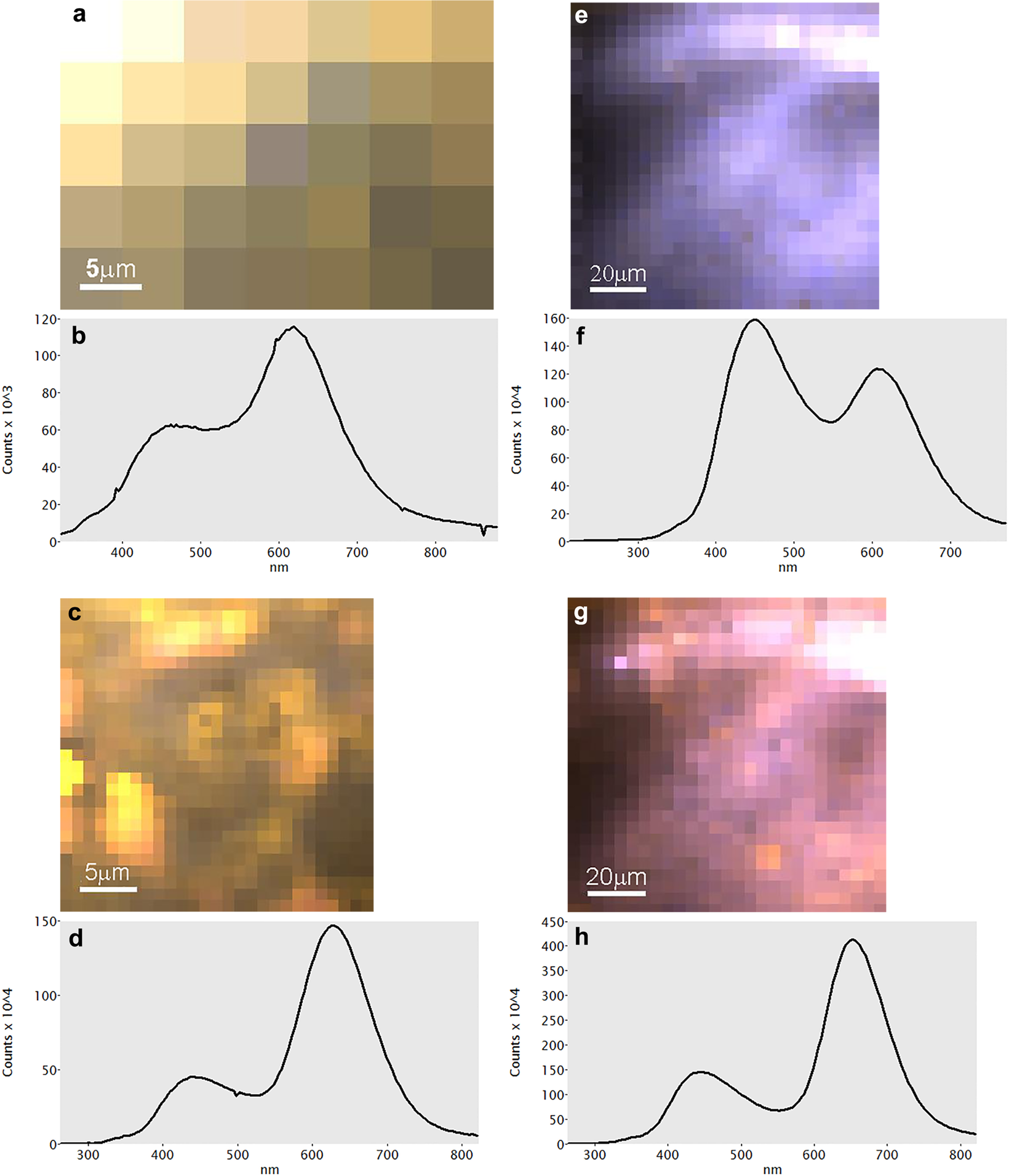
Cross-plots of major element oxide composition and calculated calcite... | Download Scientific Diagram

Difference Between Calcium and Calcium Carbonate | Chemical Properties, Occurrence, Uses, Differences
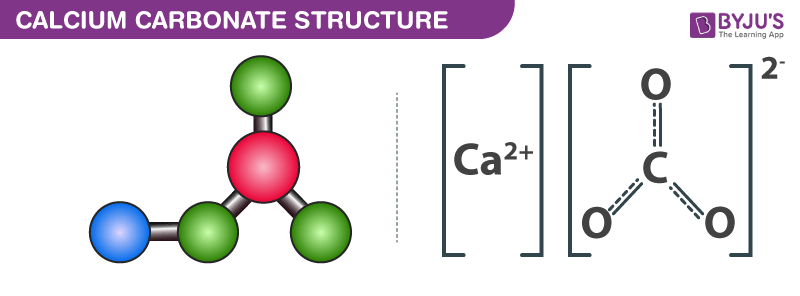
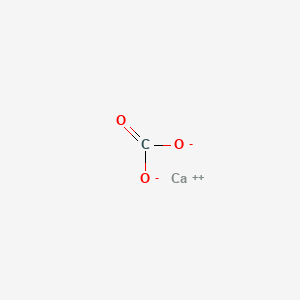
Limestone: Calcium Carbonate (CaCO3) - Uses, Preparation, Properties, Formula & Structure of Calcium Carbonate

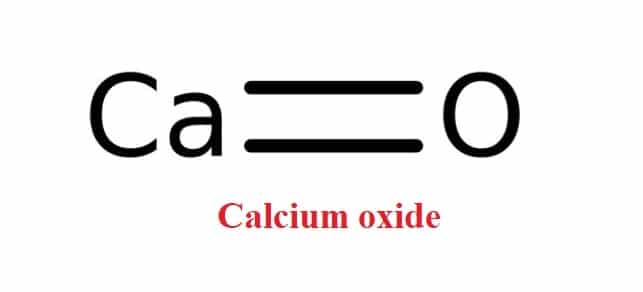
Question Video: Calculating the Mass of Calcium Carbonate Required to Produce a Given Mass of Calcium Oxide | Nagwa

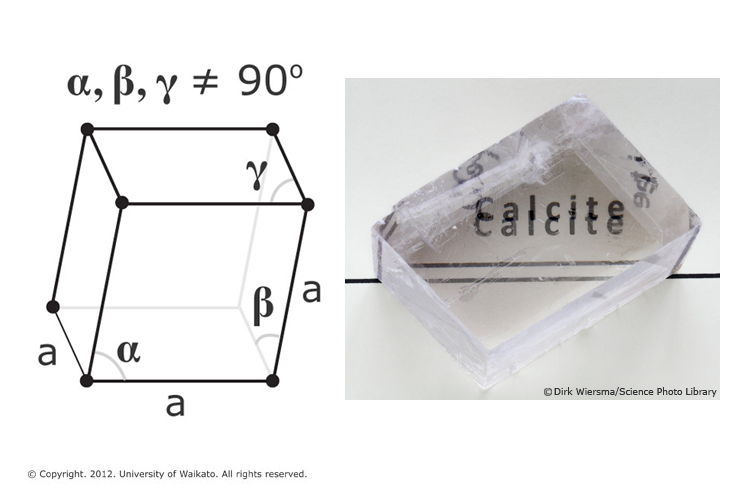
Crystals | Free Full-Text | Review and Recalculation of Growth and Nucleation Kinetics for Calcite, Vaterite and Amorphous Calcium Carbonate

DOC) IB Chemistry IA: Determining the Empirical Formula of Magnesium Oxide | Josephine Yeh - Academia.edu
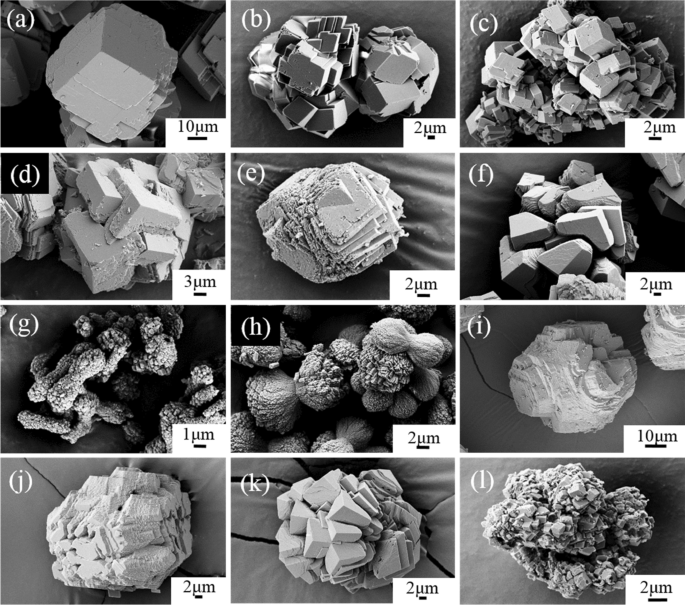
Divalent heavy metals and uranyl cations incorporated in calcite change its dissolution process | Scientific Reports

Modelling how incorporation of divalent cations affects calcite wettability–implications for biomineralisation and oil recovery | Scientific Reports
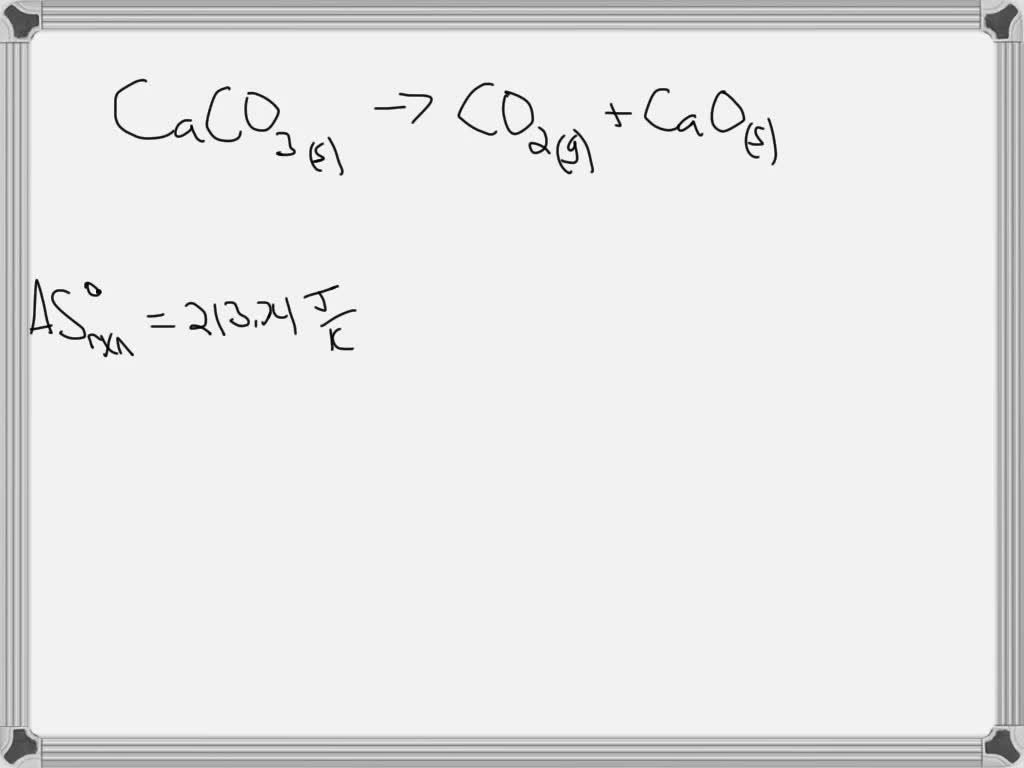
SOLVED: 12. Calculate the standard reaction entropy for the decomposition of 1 mol calcite to carbon dioxide gas and solid calcium oxide at 25°C. CaCO3 (s) â†' CO2 (g) + CaO (s)