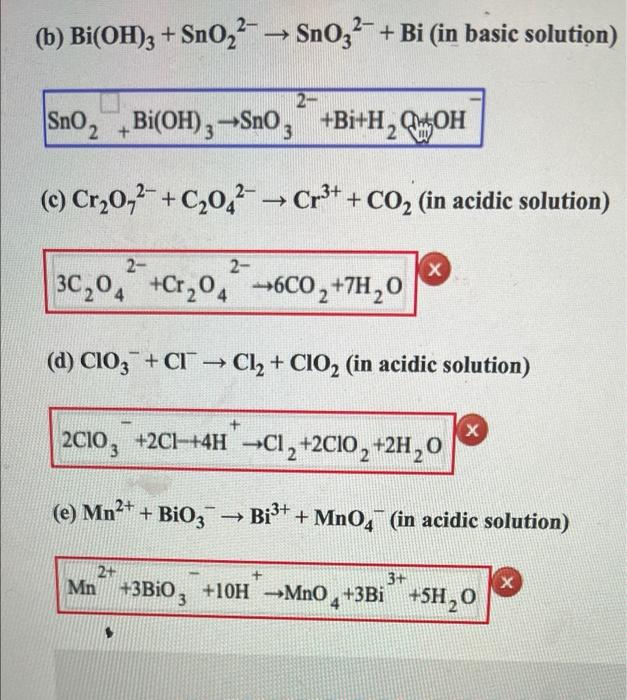
Balance the following redox equation by half reaction method : Bi(OH)3(s) + SnO^(2-)2(aq) ⟶ SnO^(2-)3(aq) + Bi(s) - Sarthaks eConnect | Largest Online Education Community

Bi(OH)3 oxidation state @mydocumentary838. find the oxidation number of bismuth in bi(oh)3. - YouTube

SOLVED: Use the rules (in order) to assign oxidation numbers to each of the elements in the compounds below: hydroxylamine NH,OH thiosulfate ion Sz03 bismuth hydroxide Bi(OH)3
A novel bismuth hydroxide (Bi(OH)3) semiconductor with highly-efficient photocatalytic activity - Chemical Communications (RSC Publishing)
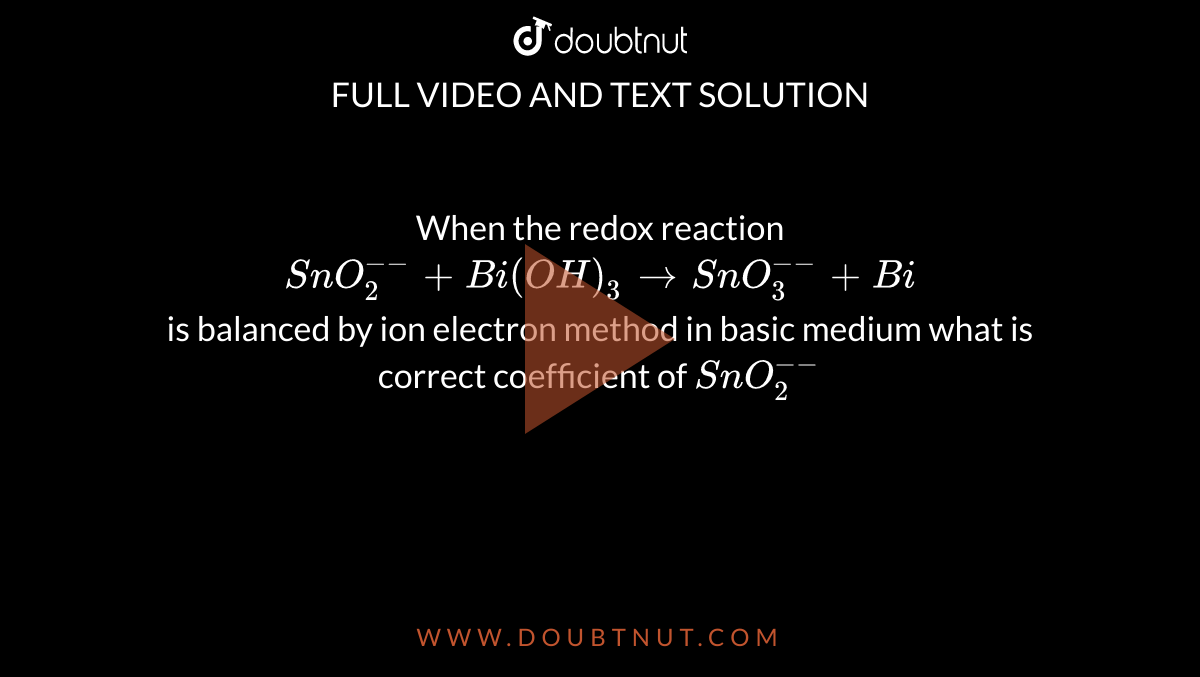
When the redox reaction SnO(2)^(--)+Bi(OH)(3)toSnO(3)^(--)+Bi is balanced by ion electron method in basic medium what is correct coefficient of SnO(2)^(--)

High toxicity of Bi(OH)3 and α-Bi2O3 nanoparticles towards malignant 9L and MCF-7 cells. | Semantic Scholar
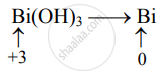
Balance the following reaction by oxidation number method. Bi(OH)X3(s)+Sn(OH )X3(aq)−⟶BiX(s)+Sn(OH)X6(aq)2−(basic) - Chemistry | Shaalaa.com

Balance the following redox reactions by ion - electron method:1. MnO4^ - (aq) + I^ - (aq) → MnO2(s) + I2(s) ( in basic medium)2. MnO4^ - (aq) + SO2(g) → Mn^2 + (aq) + HSO4^ - (aq) ( in acidic solution)

Bi(OH)3 oxidation state @mydocumentary838. find the oxidation number of bismuth in bi(oh)3. - YouTube

Separation and Identification of Bismuth and Tin Ions Experiment 6 Qualitative Analysis. - ppt download

3%20+%20NaOH%20=%20Bi(OH)3%20+%20NaNO3.svg)

.PNG)


![ANSWERED] Use the rules (in order) to assign oxidat... - Physical Chemistry ANSWERED] Use the rules (in order) to assign oxidat... - Physical Chemistry](https://media.kunduz.com/media/sug-question/raw/50803193-1659119749.0329337.jpeg)
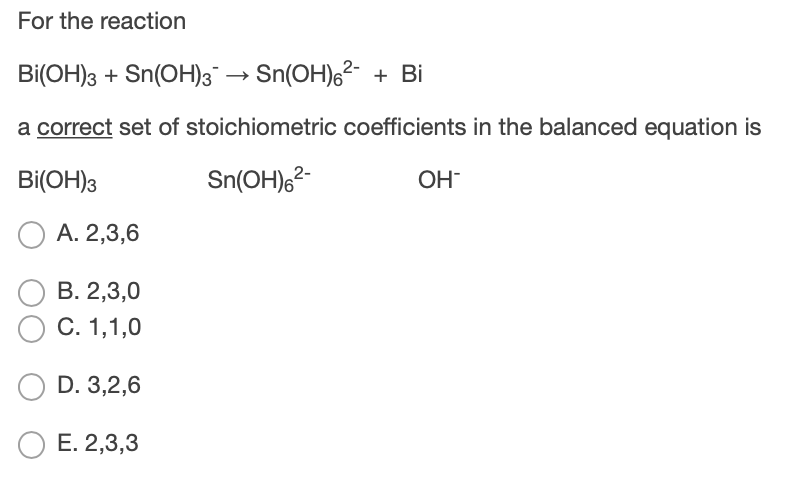

3.svg)