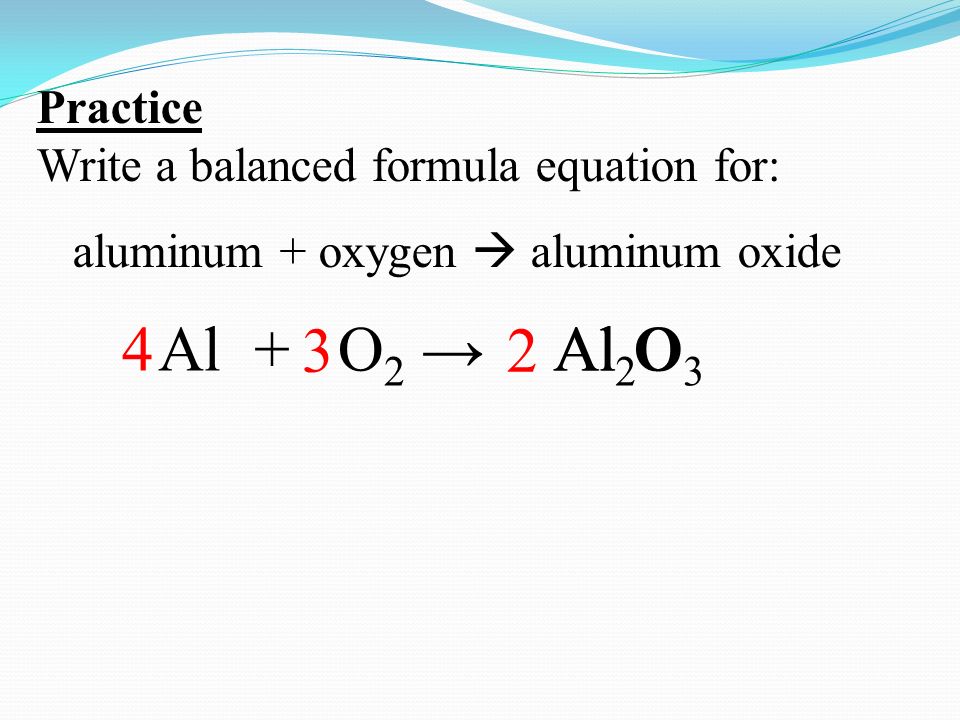
Practice Write a balanced formula equation for: aluminum + oxygen aluminum oxide Al +Al OO2 →O2 →Al 2 O ppt download

OneClass: Aluminum powder can burn in pure oxygen to form aluminum oxide, AI_2O_3. The balanced equat...

SOLVED: Solid aluminum reacts with oxygen gas to form solid aluminum oxide. If 7.9 moles of aluminum reacts with excess oxgyen gas, how many moles of aluminum oxide can be formed? (Be
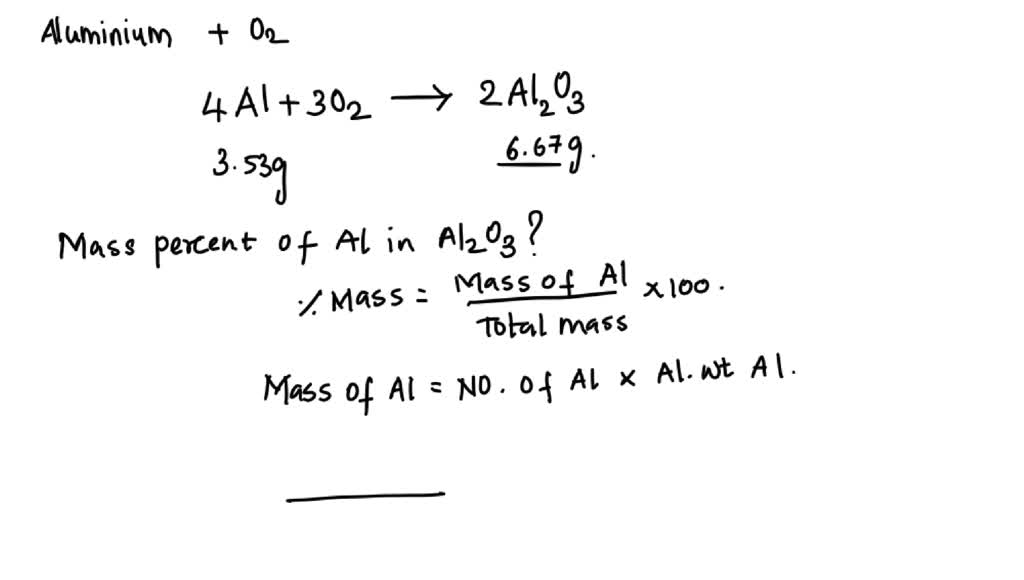
SOLVED: A 3.53-g sample of aluminum completely reacts with oxygen to form 6.67 g of aluminum oxide. Use this data to calculate the mass percent composition of aluminum in aluminum oxide.

OneClass: Aluminum and oxygen react to form aluminum oxide. 4 Al + 3 O_2 rightarrow 2 Al_2O_3 a. What...

aluminium reacts with oxygen to form aluminium oxide. how many grams of oxygen are required to react - Brainly.in
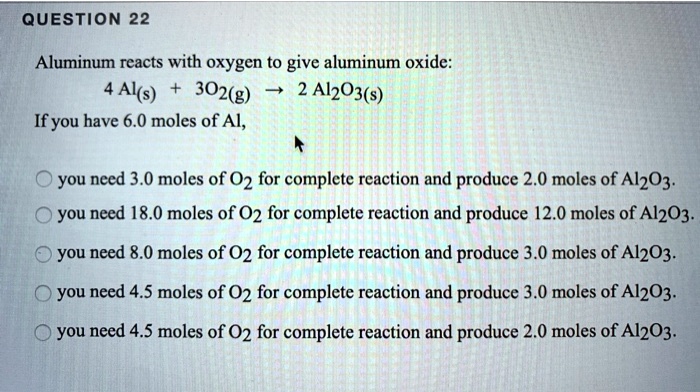
SOLVED: QUESTiON 22 Aluminum reacts with oxygen to give aluminum oxide: Al(s) 302(g) Al203(s) If you have 6.0 moles of Al, you need 3.0 moles of 02 for complete reaction and produce




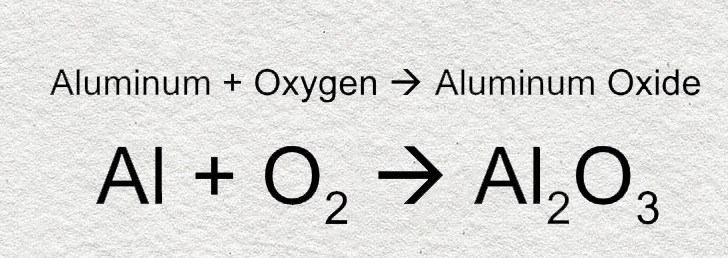
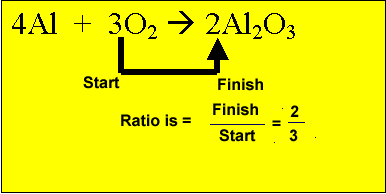
![ANSWERED] Aluminum reacts with oxygen to form alumi... - Physical Chemistry ANSWERED] Aluminum reacts with oxygen to form alumi... - Physical Chemistry](https://media.kunduz.com/media/sug-question/raw/58591902-1659703920.1450665.jpeg)






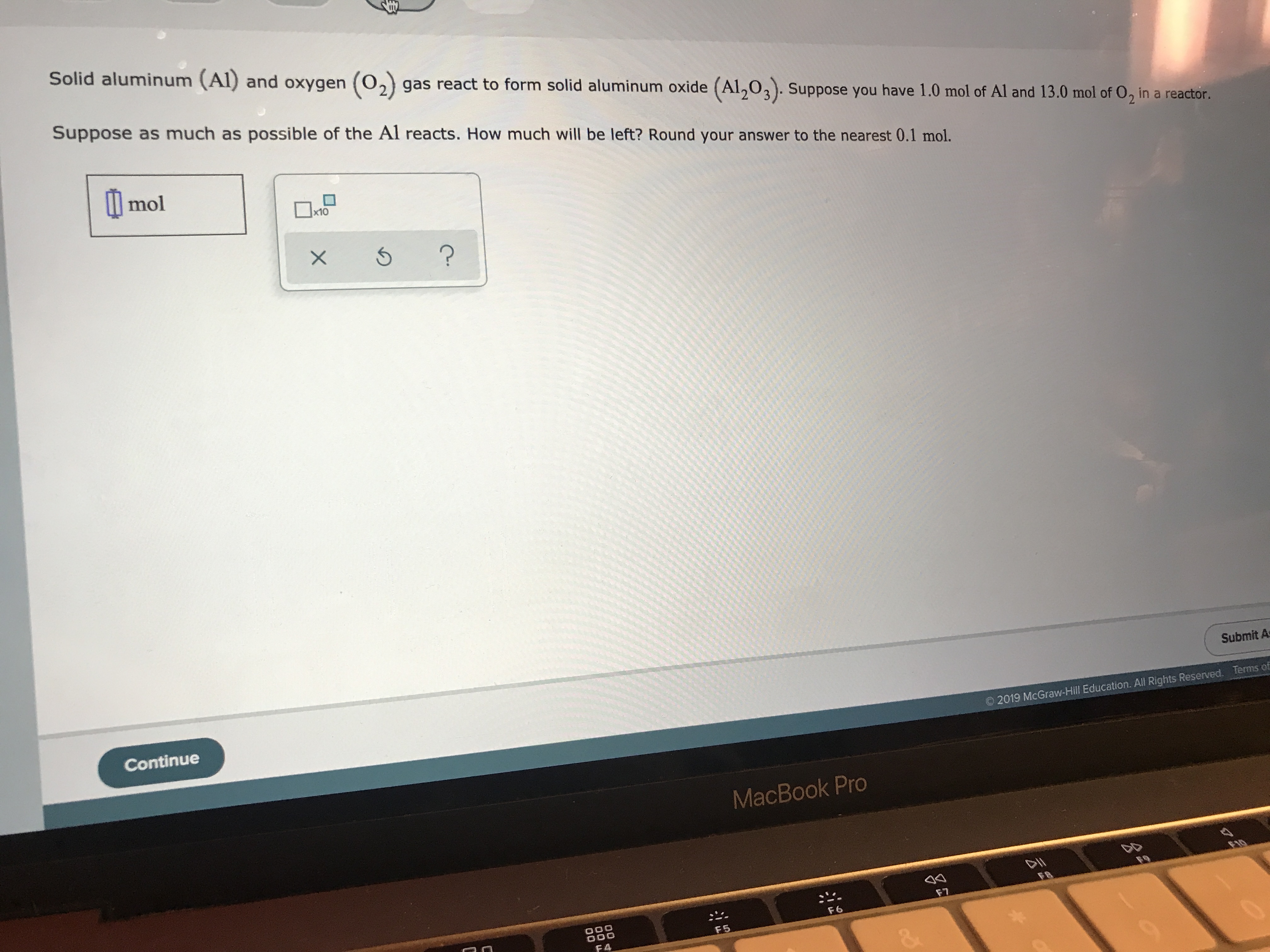
![ANSWERED] Aluminum reacts with oxygen to form alumi... - Physical Chemistry ANSWERED] Aluminum reacts with oxygen to form alumi... - Physical Chemistry](https://media.kunduz.com/media/sug-question/raw/58592057-1659703865.5915215.jpeg)