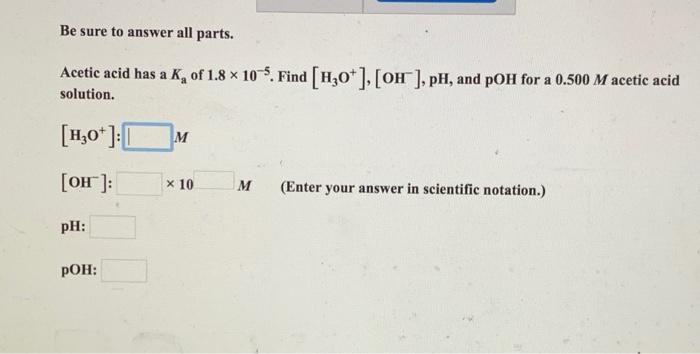
![SOLVED: Part A Acetic acid has a Ka of 1.8 X 10 5 Three acetic acid/acetate buffer solutions, A, B,and C, were made using varying concentrations: A. [acetic acid] ten times greater SOLVED: Part A Acetic acid has a Ka of 1.8 X 10 5 Three acetic acid/acetate buffer solutions, A, B,and C, were made using varying concentrations: A. [acetic acid] ten times greater](https://cdn.numerade.com/ask_previews/bece322a-fdc0-4fa6-9201-e3070264317a_large.jpg)
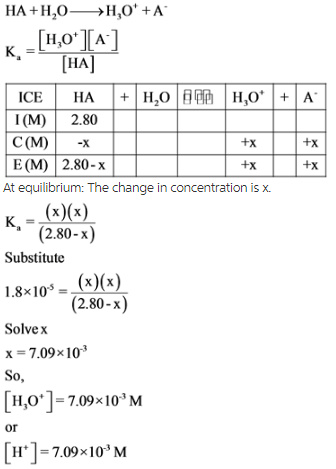
SOLVED: Part A Acetic acid has a Ka of 1.8 X 10 5 Three acetic acid/acetate buffer solutions, A, B,and C, were made using varying concentrations: A. [acetic acid] ten times greater

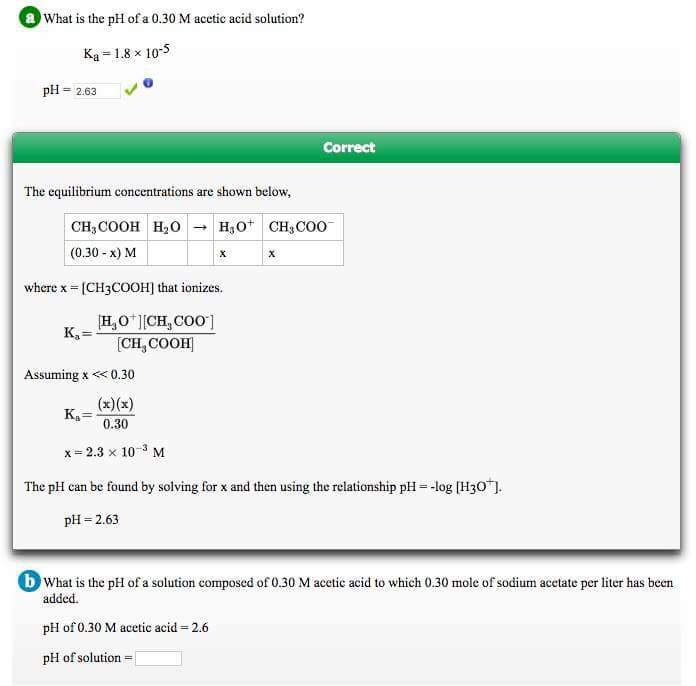
SOLVED: Part A You need to produce a buffer solution that has a pH of 5.09. You already have a solution that contains 10. mmol (millimoles) of acetic acid. How many millimoles
What is the pH of 0.1 m of acetic solution, if acetic acid is a weak acid with Ka2 1.86 × 10-5? - Quora
![The pH of 0.1 M acetic acid solution is closest to[Dissociation constant of the acid, Ka = 1.8 × 10^-5 ] The pH of 0.1 M acetic acid solution is closest to[Dissociation constant of the acid, Ka = 1.8 × 10^-5 ]](https://haygot.s3.amazonaws.com/questions/1938773_1231298_ans_7f19b0e0d221405fbf38b0df680608aa.jpg)
The pH of 0.1 M acetic acid solution is closest to[Dissociation constant of the acid, Ka = 1.8 × 10^-5 ]
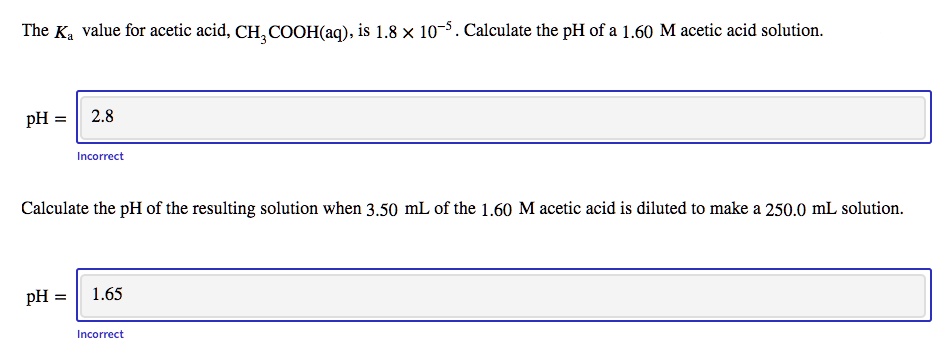
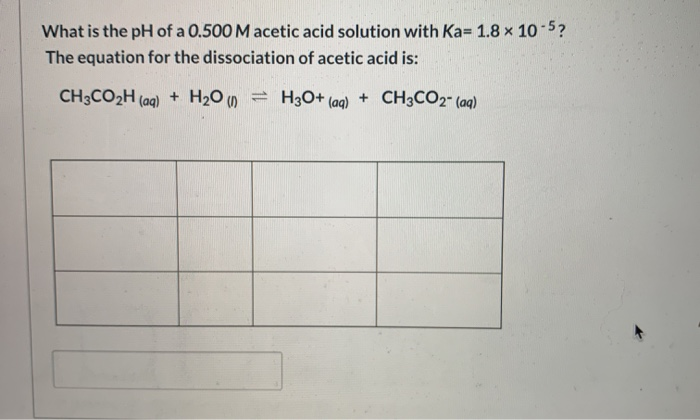
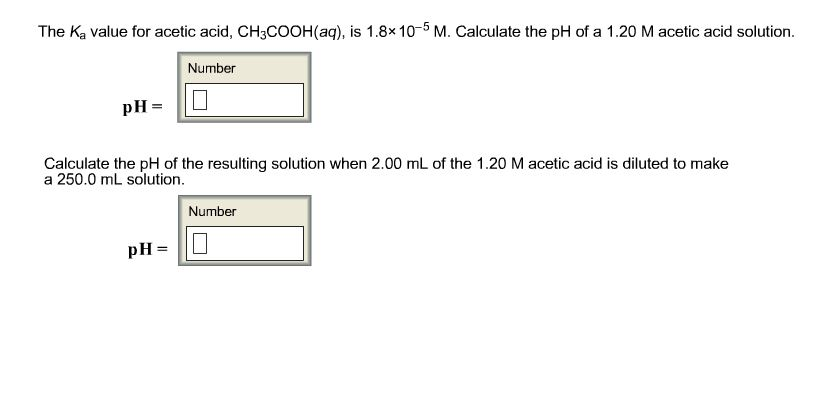
SOLVED: The Ka value for acetic acid, CH;C COOH(aq), is 1.8 x 10-5 . Calculate the pH of a 1.60 M acetic acid solution. pH 2.8 Incorrcct Calculate the pH of the
The pH of an acetic acid solution is 3.26. What is the concentration of acetic acid and what is the percent of acid that's ionized? - Quora
![Calculate pH of a buffer prepared by adding 10 mL of 0.10 M acetic acid to 20 mL of 0.1 M sodium acetate. [pKa (CH3COOH) = 4.74 ] Calculate pH of a buffer prepared by adding 10 mL of 0.10 M acetic acid to 20 mL of 0.1 M sodium acetate. [pKa (CH3COOH) = 4.74 ]](https://d1hhj0t1vdqi7c.cloudfront.net/v1/dDlCNVZnUE9URzQ=/sd/)
Calculate pH of a buffer prepared by adding 10 mL of 0.10 M acetic acid to 20 mL of 0.1 M sodium acetate. [pKa (CH3COOH) = 4.74 ]